Next Generation Battery Systems...
What are the right systems to succeed the Lithium Ion Battery...? All of this is clearly fundamental research, but as synthetic chemist, one can always think on possible reaction that could work as reversible battery reactions. Currently our group works on using Mg, Ca and Al as an Anode as well as sulfur or organic materials as the cathode. In addition, we have worked out an all maganese redox flow battery.
Aluminium-Battery: With aluminium being the most abundant metal in Earth’s crust, rechargeable Al ion batteries (AIBs) hold great promise as next-generation energy storage devices. Here, we present an organic redox polymer with two well-defined redox processes as a positive electrode material that reversibly inserts [AlCl4]− ions and delivers specific capacities of up to 167 mAh g−1. After 5,000 cycles at a 10 C rate this AIB retains 88% of its capacity. Even at a 100 C rate, 64 mAh g–1 can be reversibly cycled, and the AIB returns unchanged to its original capacities at slower rates. This AIB constitutes a major advance in the development or rechargeable AIBs and will initiate further explorations of organic redox polymers as positive electrode materials, paving the way towards more sustainable energy storage solutions. Read more on this collaborative work with Prof. B. Esser now at Ulm University in:
"On a high-capacity aluminium battery with a two-electron phenothiazine redox polymer as positive electrode." G. Studer, A. Schmidt, J. Büttner, A. Fischer, I. Krossing*, Birgit Esser*, Energy Environ. Sci. 2023, 16, 3760-3769. https://doi.org/10.1039/D3EE00235G
Due to the very favorable referee reports, this publication was selected as a title page of the journal. It reached a huge Altmetric attention score of 173 (https://rsc.altmetric.com/details/148802594#score) and is in the 99th percentile or the top 5% of all research outputs ever tracked by Altmetric.

Too much fluorine? Weakly coordinating anions improve electrolytes for magnesium ion batteries by inhibiting ion-pairing, increasing the electrochemical window and conductivity. A novel synthetic route towards clean and contaminant-free [Mg(L)x][Al(ORF)4]2 (L =MeCN, DME; x = 3;6; RF = C(CF3)3), containing [Al(ORF)4]− (also called [TPFA]−, [Al(OtBuF]4−, [pf]−) as one of the least coordinating anions, led to surprising electrochemical data, contradicting previously published results using this electrolyte salt in DME solution.
(Batteries & Supercaps 2022, e202200340. https://doi.org/10.1002/batt.202200340)
All Mn-Redox Flow Battery
A new all‑Manganese flow battery (all‑MFB) as non‑aqueous hybrid redox‑flow battery is reported. The discharged active material [Cat]2[MnIICl4] (Cat = organic cation) utilized in both half‑cells supports a long cycle life. The reversible oxidation of [MnIICl4]2– to [MnIIICl5]2– at the positive electrode and manganese metal deposition from [MnIICl4]2– at the negative electrode give a cell voltage of 2.59 V. Suitable electrolytes were prepared and optimized, followed by a characterization in static battery cells and in a pumped flow‑cell. Several electrode materials, solvents and membranes were tested for their feasibility in the all‑MFB. An electrolyte consisting of [EMP]2[MnCl4] and some solvent g-butyrolactone was cycled 500 times, both in a static as well as a flow‑cell, over a period of two months, with coulombic efficiencies up to 83 %. With the electrolytes prepared in this work, energy densities up to 74 Wh L–1 are possible, exceeding the VRFB benchmark system, using solely the cheap and abundant element manganese as the active material. Although further optimizations are necessary, this system describes a new and promising setup towards sustainable stationary energy storage.
Advanced Energy Materials 2021, 11, 2101261.


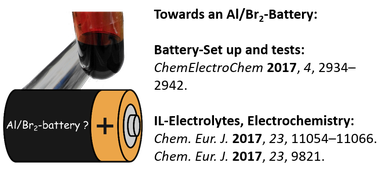
First investigations towards the feasibility of an Al/Br2-battery (see picture) based on bromoaluminate and polybromide ionic liquids are presented. The battery exhibited an open circuit voltage of 1.1 V and a theoretical energy density of 33 Wh L–1 based on the migration of cations, which was determined by NMR studies. The battery could be discharged with high resistance values, while preliminary charging attempts revealed high over potentials.
