Concept to Unified Acidity and Reducity Scales...
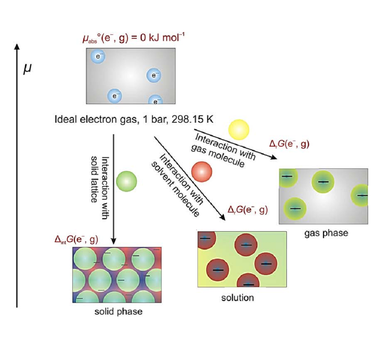
Acid-base and redox chemistry are the two most fundamental concepts in general chemistry. Both are taught already in school. However, both concepts are usually limited to one medium/solvent, although they are related through the gaseous hydrogen atom delivering the proton for Brønsted acidity and the electron for redox chemistry. In our work we describe, how the ideal proton gas and the ideal electron gas form the reference states for the absolute pH Brønsted acidity scale and the absolute pe redox scale.
Review Perspective: Angew. Chem., Int. Ed. 2018, 57, 4386-4411. This review aims at explaining the concept.
Video Tutorials: In the section 'Video Tutorials' of this Website you will find a series of videos explaining the concept. So go ahead and use it...! There are myriads of possible applications.